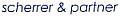Blog
Biohazardous Waste Decontamination in an Autoclave
Authors: Aaron Mertens, Marcel Dion
Pharmaceutical and biotechnology manufacturing, laboratory and research facilities are required to decontaminate biological waste prior to disposal. In most cases, autoclaves (also referred to as steam sterilizers) are utilized to accomplish this task. Autoclave performance is affected by temperature at the actual materials being treated, so air removal and steam penetration are critical parameters. These can be influenced by loading and cycle design; and they can be tested using chemical indicators for time at temperature, and biological indicators for performance treating a reference organism. This article is meant to provide guidance and recommendations to ensure optimal and reliable performance of the decontamination process using autoclave steam sterilizers.
Biohazardous waste decontamination cycle parameters in an autoclave are typically set at 121oC for > 60 minutes or 132oC for > 30 minutes. Actual temperatures within the autoclave chamber and load can be verified using chemical indicators, specifically those which change color after exposure to saturated steam for a specific time and temperature. This is important, as the autoclave set point temperature may not reflect the temperature achieved within the chamber, especially with a dense load. Decontamination cycles typically require a longer sterilization time due to the size / density of the load and challenges in air removal / steam penetration. Therefore, the chemical indicator should be placed at the most difficult to sterilize location (center of densest location in load, for example) and also throughout the chamber to verify adequate steam penetration and uniform temperature distribution.
Preparation and placement of the biodecontamination bags is critical for successful sterilization. Testing has demonstrated that bags should not be tightly sealed, as this can inhibit steam penetration into the bags, resulting in temperatures below 121oC. A recommendation is to leave “two fingers” width in the opening of the bag when tying.
Figure 1 illustrates how the temperature increases in the middle of a biohazard bag during the decontamination cycle when the bag is completely closed. One can see that even after 55 minutes of exposure, the temperature in the bag has not reached sterilization temperature of 121oC. This is due to the combined effects of air not being completely removed from the bag and steam not effectively penetrating inside the bag.
Figure 1. Temperature in zip tied closed biohazard bag
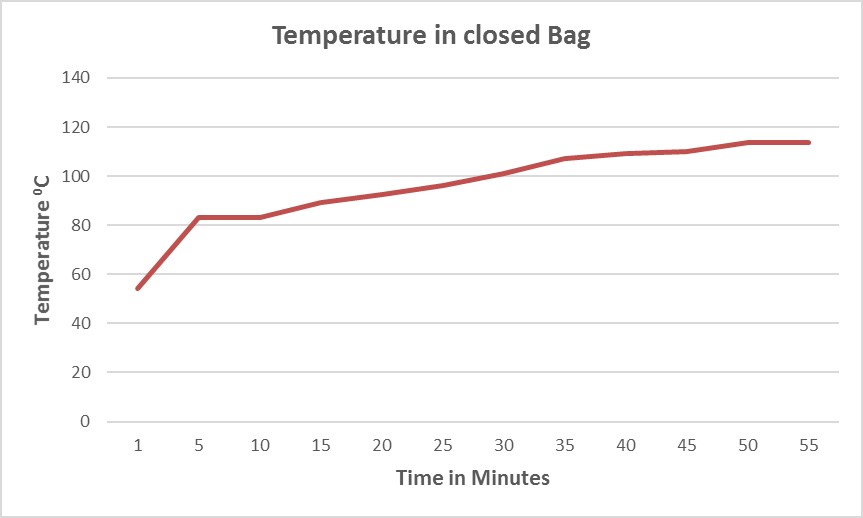
Figure 2 shows that by following the “two fingers” rule, the temperature inside the same bag (actually with a slightly larger load) can reach the sterilization set point within a significantly shorter period of time (approximately 37 minutes) because steam can more easily penetrate inside the bag.
Figure 2. Temperature in partially open biohazard bag
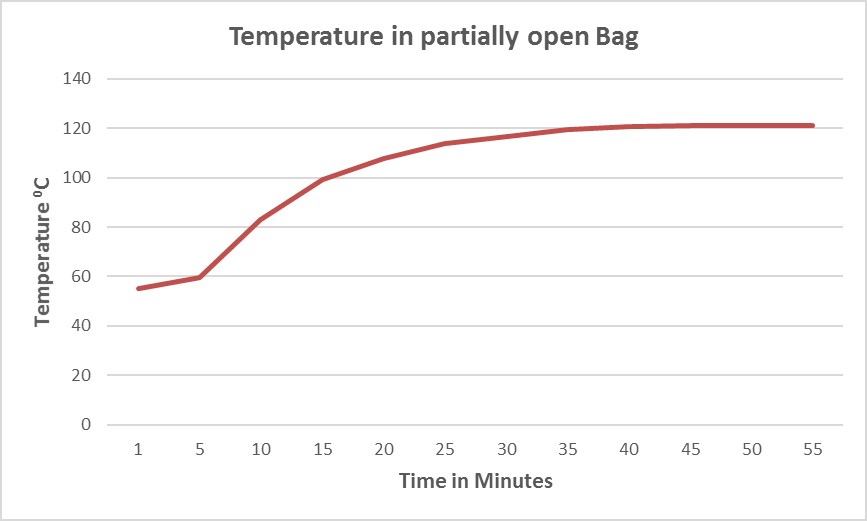
Since air removal and steam penetration are challenges for decontamination of densely packed biohazard bags, autoclave cycle parameters should be optimized to account for this. Figure 3 demonstrates how the use of short vacuum pulses at the beginning of the cycle can drastically reduce the heat up time by more effectively removing air from the partially open bags and pulling steam inside.
Figure 3. Optimized cycle for biohazard bags
Additionally, there needs to be sufficient space around the bags to allow for air removal and steam penetration throughout the load. Overpacking the autoclave with too many waste bags can prevent effective air removal and steam penetration. Sometimes plastic or metal trays are used to contain waste which becomes liquefied in the autoclave. This can be an effective technique, but should be part of the controlled loading pattern. For example, if trays are used, the tray depth must be minimized to ensure air proper air removal.
Biological indicators are typically used to demonstrate microbiological kill. For steam cycles, the organism used for the biological indicator is Geobacillus stearothermophilus. This organism is accepted within the industry as very difficult to kill and is the most commonly used indicator organism for steam sterilization validation studies. Typically, the biological indicator will have a population of at least 106 organisms. At a minimum, the biological indicator should be placed at the most difficult to sterilize location within the load. It could be beneficial to place additional biological indicators throughout the autoclave chamber, perhaps adjacent to a chemical indicator, to demonstrate that saturated steam is uniform throughout the autoclave chamber.
The self-contained biological indicator (SCBI) is recommended for validation of a decontamination cycle. The SCBI should include the organism Geobacillus stearothermophilus at a population of approximately 1x106. The SCBI does not need to be handled aseptically between processing and incubation, which is advantageous when a microbiology lab with aseptic conditions is not available.
Chemical indicators can be used for steam exposure time and temperature verification. A Type 4 Multivariable Indicator, per ANSI/AAMI/ISO 11140-1, is recommended for monitoring a biohazardous waste decontamination cycle. The Type 4 Indicator is designed to react to two or more critical variables (e.g. time and temperature) during the sterilization cycle.
It is imperative to define and test the cycle parameters (air removal, sterilization time, sterilization temperature, post sterilization conditioning, vacuum, drying, cooling) used for decontamination. Further, the load pattern (number of bags, bag preparation, trays, etc) should be specified and controlled. These elements should be tested using thermocouples, chemical indicators and biological indicators routinely to demonstrate sterilization conditions are successfully achieved. Also, autoclave cycle printouts should be reviewed following the sterilization cycle completion to ensure critical parameters have been met for each decontamination cycle processed.
About the Authors:
Aaron Mertens has been a member of the STERIS Life Sciences Contamination Control Solutions as a Technical Service Specialist since January 2015. In this role, Aaron has responsibility for providing global technical support primarily for Critical Environments, Sterility Assurance and Barrier Products, application and validation.
Previously, Aaron has held a number of positions within the Pharmaceutical Industry, specializing in cleaning, disinfection, sterilization and contamination control in parenteral drug manufacturing. In these roles, he has gained experience interfacing with industry regulatory agencies (FDA, EMEA, Japan), representing quality assurance programs.
Aaron has been a member of various industry organizations (PDA, ISPE) since 1999, contributing by presenting posters and talks at meetings, as well as participating in local chapter functions. He holds a bachelor’s degree in Genetics from the University of Wisconsin – Madison.
Marcel Dion is Director of Marketing for Washing and Steam Sterilization Systems in the Life Sciences Division of STERIS Corporation. Marcel holds a diploma in instrumentation and control from Levis-Lauzon CEGEP in Quebec, Canada. He was involved in designing and manufacturing washing systems for the Life Sciences industry during the first 20 years of his career.
For the past 17 years, he has been responsible for developing and bringing to the market innovative and efficient cleaning and steam sterilization systems for critical parts/components that are involved in the drug manufacturing process. Marcel has been a member of ISPE, PDA, LAMA and AALAS organizations for several years.
 |
Aaron J. Mertens Technical Service Specialist |
 |
Marcel Dion Director of Marketing |
Es freut uns, wenn Sie diesen Blogbeitrag kommentieren. Kommentar erfassen 182 Gefällt mir 4890